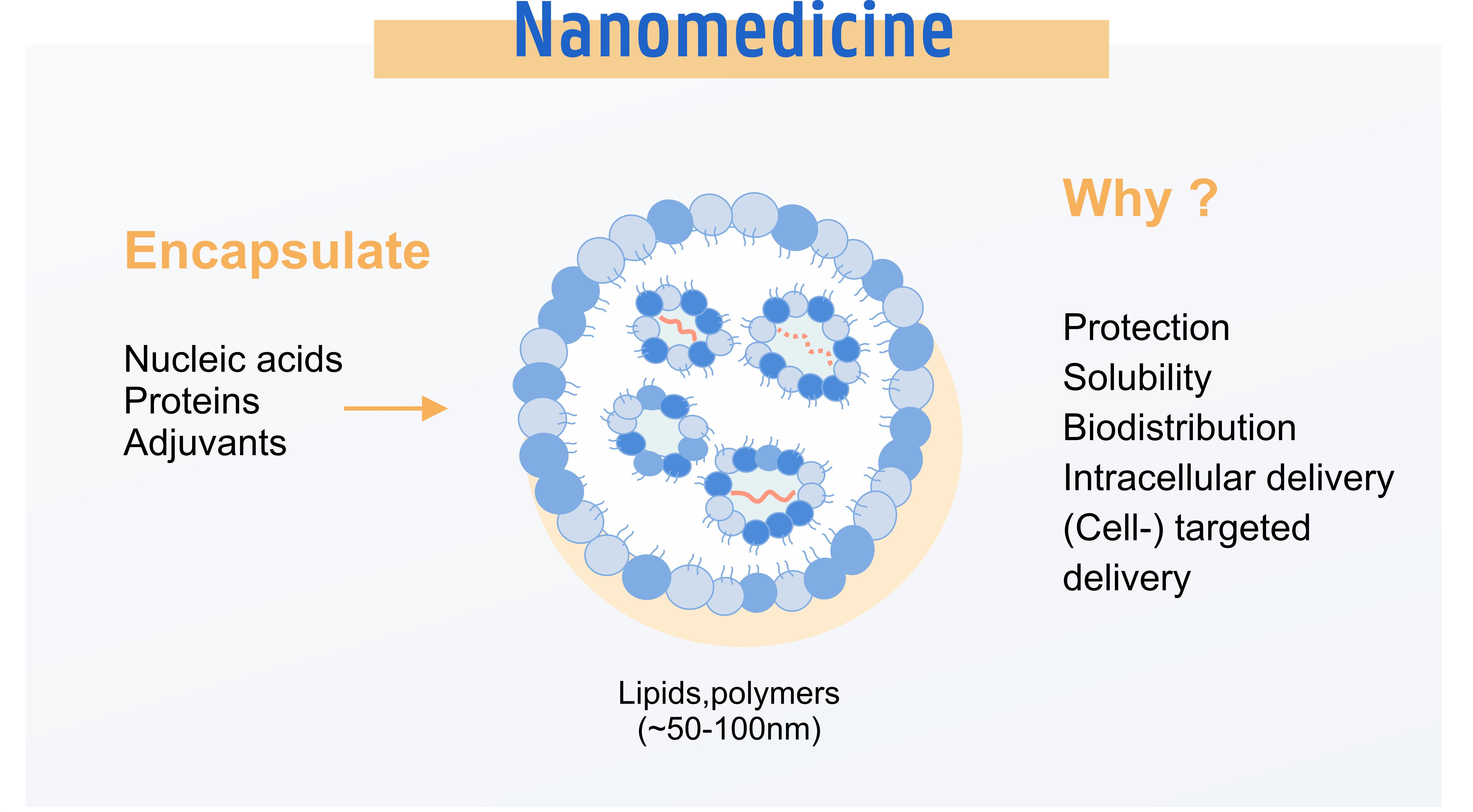
Characterization of Nanomedicines
NMC is establishing a validated toolbox that integrates cutting-edge assays for a comprehensive characterization of critical parameters to the development, production and quality control of nanomedicines.
Advanced analytical techniques for understanding the stability and heterogeneity of nanomedicines; e.g. AF4, Cryo-EM.
GMP compliant methods for measuring particle size-, zeta potential, lipid identity and mRNA integrity.
Biological evaluation of nanomedicines
VaxAdvance
VaxAdvance unites four leading entities:
NMC: Formulation, preclinical development, QC testing
CellGENTherapies: GMP production, QC testing, IMPD writing
Core ARTH Animal Facilities: Advanced infrastructure, services and expertise for conducting preclinical (bio)medical (laboratory animal) research
Center for Vaccinology (CEVAC): Clinical trail unit, Immuno-monitoring
Together providing comprehensive services for developing and producing clinical batches of next-generation vaccines, including mRNA and saRNA platforms.
Our goal is to accelerate breakthroughs in vaccine and therapeutic development from the lab bench to real-world clinical applications.
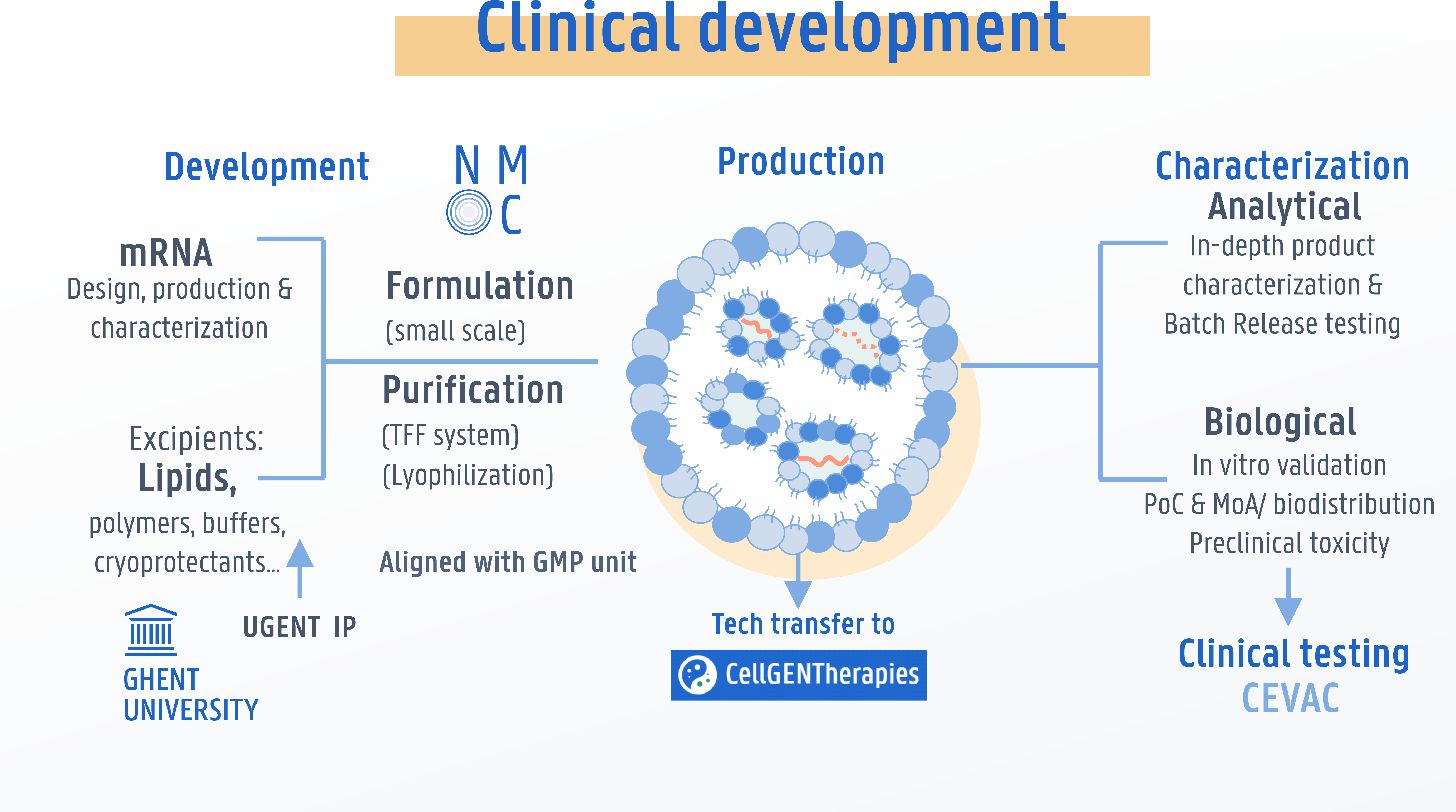
Services
NMC is open for collaboration with academic research groups and companies on a fee-for-service basis or as partner in R&D co-development projects. A dedicated team is on-hand to develop, test and optimize nanomedicine products and support with technical problem-solving, assay development and knowledge on GMP and regulatory processes.
Formulation

Encapsulation of
nucleic acids and other
biotherapeutics in state-of-
the-art liposomes or
lipid nanoparticles (LNPs).
Characterization

Analytical- and biological
characterization of
nanomedicines,
including QC in GMP.
Translation

Supporting the transition of
nanomedicines
from early concept to
first clinical testing